Sars-cov-2 - Tackling the Mysteries of the Long
Science Brief: Community Use of Cloth Masks to Control the Spread of SARS
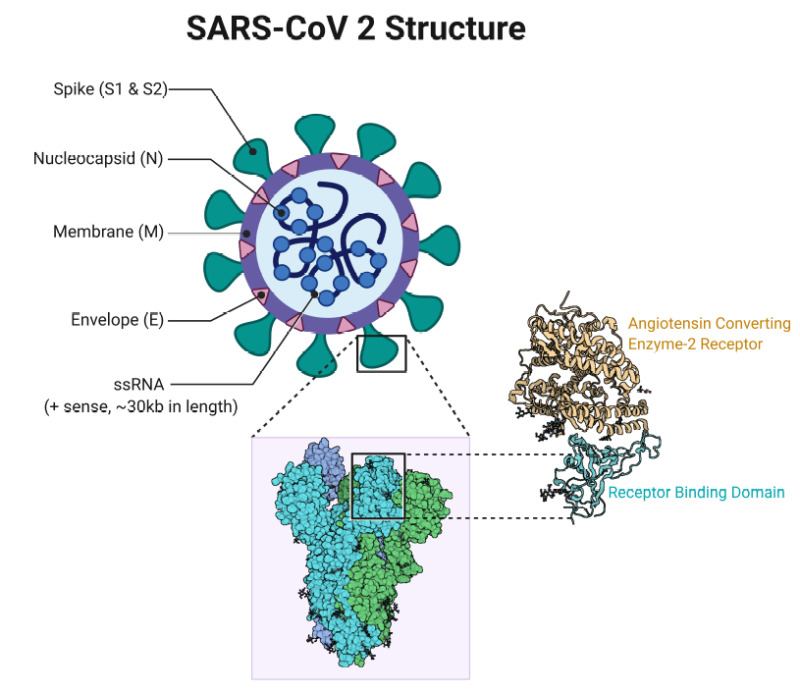
There have been a few reports of severe allergic reactions following SARS-CoV-2 vaccination, including some reports of patients who experienced anaphylaxis after receiving a SARS-CoV-2 mRNA vaccine. Boulware DR, Pullen MF, Bangdiwala AS, et al. Bahl P, Bhattacharjee S, de Silva C, Chughtai AA, Doolan C, MacIntyre CR. de Oliveira PM, Mesquita LC, Gkantonas S, Giusti A, Mastorakos E January 2021. Methods: Non-systematic review. Morris SB, Schwarts NG, Patel P, et al. Denny TN, Andrews L, Bonsignori M, et al. Recommendations for Clinical Laboratory Staff and Health Care Providers Using This Test Be aware that one positive target and one negative target showing the S-gene drop out reduced sensitivity with the S-gene target when using the Linea COVID-19 Assay Kit is consistent with certain mutations, including those in the B. Guy 51 Population-based intervention with trend analysis 2,313 counties, US March-December County population Mandatory mask wearing in public Estimated overall initial daily decline in new diagnoses of 0. Lyu and Wehby 46 Population-based intervention with trend analysis 15 US states and Washington DC March- May State population Mandatory mask wearing in public Estimated overall initial daily decline in new diagnoses of 0. Considerations for SARS-CoV-2 Testing in Different Scenarios Diagnostic Testing Testing persons with signs or symptoms consistent with COVID-19 Positive test results using a viral test NAAT or antigen in persons with signs or symptoms consistent with COVID-19 indicate that the person has COVID-19, independent of vaccination status of the person. In SARS-CoV-2 the recognition site is formed by the incorporated 12 CCT CGG CGG GCA which corresponds to the amino acid sequence. Due to the significance of asymptomatic and pre-symptomatic transmission, this guidance further reinforces the need to test asymptomatic persons, including of a person with documented SARS-CoV-2 infection. Sickbert-Bennett EE, Samet JM, Prince SE, et al. Behera P, Patro BK, Singh AK, et al. This name was chosen because the virus is genetically related to the coronavirus responsible for the SARS outbreak of 2003. At homes, places of work, nursing homes, prisons, and others. About the National Institutes of Health NIH : NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U. An example of public health surveillance testing is when a state public health department develops a plan to randomly select and sample a percentage of all people in a city on a rolling basis to assess local infection rates and trends. Among the variant's several mutations is one in the receptor-binding domain of the spike protein that changes the asparagine at position 501 to tyrosine N501Y. Surveillance testing is primarily used to gain information at a population level, rather than an individual level. Virus infections start when viral particles bind to host surface cellular receptors. The boxed region in A is reconstructed by projecting the solved postfusion S structure onto the refined coordinates. Whether they include of ACE2, as seen in similar coronaviruses, remains under investigation as of May 2020. Mid-turbinate nasal swabs were collected daily during the first 14 days, with the primary endpoint being PCR-confirmed SARS-CoV-2 infection within 14 days after enrollment in those who were not infected at baseline. Among the 97 participants, only 17 were confirmed to be SARS-CoV-2 PCR positive. Interim considerations: preparing for the potential management of anaphylaxis after COVID-19 vaccination. At the time of the second interim analysis when 125 of 132 participants who provided consent were evaluable for the primary endpoint , the Data Safety Monitoring Board recommended early termination of the study for futility. Jun 27 2020;395 10242 :1973-1987. Klompas M, Baker MA, Rhee C 4 August 2020. 12 Study Population• 100924• The latest clinical trials for SARS-CoV-2 PEP can be found at. CS1 maint: PMC format• , high number of false positives when used in a community where prevalence of infection is low. The native conformation of the ribonucleoproteins RNPs and their higher-order assemblies were revealed. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Freedman DO, Wilder-Smith A. D The best solved domains of the RBD down S, HR1 and CH from the S2 subunit, are highlighted with the fitted PDB: 6XR8. from the original on 28 February 2020. ; Cobey, Sarah; Deverman, Benjamin E. , NIAID Director and Chief, Laboratory of Immunoregulation, is available to comment on this research. See also Figure S1 and Video S1. Screening can be particularly helpful in certain scenarios see examples below , especially when testing is done serially and in areas with substantial or high levels of community transmission Table 2 or in the setting of outbreaks. The Panel recommends against the use of other drugs for SARS-CoV-2 PEP, except in a clinical trial AIII. However, the study provided no data on the characteristics of the study participants, types of exposures, or how endpoints were defined. 51 also demonstrated reductions in mortality. Participants were recruited using online advertising, social media, and referrals from hospitals, health departments, and individuals with laboratory-confirmed SARS-CoV-2 infection. Using current models of age-dependent infection fatality rates, upper and lower limits for the attack rate in Germany can be estimated between 0. The study excluded individuals who were allergic to hydroxychloroquine and those with glucose-6-phosphate dehydrogenase deficiency, retinal disease, or substantial cardiac disease. , 20-30 microns and larger 9 but they can also block the exhalation of fine droplets and particles also often referred to as aerosols smaller than 10 microns ; 3 , 5 which increase in number with the volume of speech 10-12 and specific types of phonation. Working with HealthVerity and Aetion, NCI aggregated and analyzed patient information collected from multiple sources, including five commercial labs including Quest Diagnostics and Labcorp , electronic medical records, and private insurers. All persons being tested, regardless of results, should receive counseling on the continuation of that help prevent the transmission of SARS-CoV-2 e. The man's second infection was symptomatically more severe than the first infection, but the mechanisms that could account for this are not known. Common adverse events included gastrointestinal events, nervous system disorders, myalgia, fatigue, and malaise. Potential Impact: While the impact does not appear to be significant, the FDA is providing this information out of an abundance of caution. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. 010• Rothamer DA, Sanders S, Reindl D, Bertram TH. 17,18 A small cohort study without a control group suggested that hydroxychloroquine might reduce the risk of SARS-CoV-2 transmission to close contacts. Screening allows early identification and isolation of persons who are asymptomatic, presymptomatic, or have only mild symptoms and who might be unknowingly transmitting virus. PMID: 33034020• The Journal of Physical Chemistry Letters. It is currently spread globally. Although several studies have reported potentially promising results, the findings are limited by the design of the studies, their small sample sizes, and lack of details regarding the safety and efficacy of ivermectin. D Lipid bilayer density appears, when all RNPs are aligned and averaged using a large spherical mask. Acute heart failure in multisystem inflammatory syndrome in children MIS-C in the context of global SARS-CoV-2 pandemic. Hydroxychloroquine as Pre-Exposure Prophylaxis for COVID-19 in Health Care Workers: A Randomized Trial COVID PREP Study This double-blind, placebo-controlled, randomized clinical trial investigated whether hydroxychloroquine 400 mg given once- or twice-weekly for 12 weeks can prevent SARS-CoV-2 infection in health care workers at high-risk of exposure. The findings had the implications that may not eliminate the virus if reinfection is not an uncommon occurrence and that may not be able to provide lifelong protection against the virus. In particular, the findings come from a scientific interpretation of real-world data, which are subject to biases that may be better controlled for in a clinical trial. There have been about 96,000 confirmed cases of infection in mainland China. Joo H, Miller GF, Sunshine G, et al. Each analysis demonstrated that, following directives from organizational and political leadership for universal masking, new infections fell significantly. Coronaviruses infect humans, other mammals, and avian species, including livestock and companion animals. MIS-C and MIS-A are rare but severe immune responses to SARS-CoV-2 that can entail cardiovascular complications. Manufacturer: Applied DNA Sciences, Inc. 6 percentage points for adults aged 18—64 years after mandate implementation, compared with growth rates during the 4 weeks preceding implementation of the mandate. Table: Summary of studies that have assessed the effect of mask mandates on COVID-19 infection risks Type of investigation Location Study months all 2020 Population studied Intervention Outcome Hendrix 36 Cohort study Hair salon in Springfield, MO USA May 2 symptomatically infected stylists and 139 patrons Universal masking in salon by local ordinance and company policy No COVID-19 infections among 67 patrons who were available for follow-up Payne 39 Cohort study USS Theodore Roosevelt, Guam USA March 382 U. However, the FDA is providing this information out of an abundance of caution. Interpretation The hydroxychloroquine regimen used for PEP in this study did not prevent SARS-CoV-2 infection in healthy individuals who were exposed to a PCR-positive case. NIAID People who have had evidence of a prior infection with , the virus that causes COVID-19, appear to be well protected against being reinfected with the virus, at least for a few months, according to a newly published study from the National Cancer Institute NCI. The FDA will update this page as significant new information becomes available. The prevention benefit of masking is derived from the combination of source control and wearer protection for the mask wearer. Based on local circumstances and resources, , including the use of a test-based strategy. 5 In February 2021, FDA issued an EUA for a human adenovirus type 26 Ad26 vectored vaccine, Ad26. PMCID:• Enrollment included 829 participants from 671 households; 407 participants in 337 households received hydroxychloroquine, and 422 participants in 334 households received ascorbic acid. Li D, Jin M, Bao P, Zhao W, Zhang S May 2020. Adults with more severe illness or who are immunocompromised may remain infectious up to 20 days or longer after symptom onset, so a test-based strategy could be considered in consultation with infectious disease experts for these people. Anfinrud P, Stadnytskyi V, Bax CE, Bax A May 2020. COVID testing: One size does not fit all. Limitations• The stylists and all clients universally wore masks in the salon as required by local ordinance and company policy at the time. C Distribution of the postfusion Ss. There were no significant differences for any of the secondary efficacy endpoints among the three groups. Please check for the latest information. In other cases, new symptoms and findings are reported that appear linked to the timing of acute infection, but they only emerge afterwards and evolve over time. Similarly, a study of ninety-four patients hospitalized in January and February 2020 estimated patients shed the greatest amount of virus two to three days before symptoms appear and that "a substantial proportion of transmission probably occurred before first symptoms in the ". 11 Pre-Exposure Prophylaxis• Therefore, they identified 2019-nCoV as a virus of. Beeching NJ, Fletcher TE, Fowler R 22 May 2020. 15 Participants who were epidemiologically linked to a PCR-positive COVID-19 case were defined as study clusters called rings. Public health surveillance testing is intended to monitor community- or population-level outbreaks of disease, or to characterize the incidence and prevalence of disease. Choosing a Test When choosing which test to use, it is important to understand the purpose of the testing e. Negative test results in persons with known SARS-CoV-2 exposure suggest no current evidence of infection. It is unclear which of these mechanisms plays a key role in transmission of COVID-19. Conclusions: Despite a rapid worldwide spread, attack rates have been low in most regions, demonstrating the efficacy of control measures. To continue to comprehensively address this important research question, NCI is supporting clinical studies that monitor infection rates in large populations of people whose antibody status is known. With this support, the agency has taken swift action to develop a trans-NIH, multi-disciplinary research agenda and is announcing the. from the original on 21 March 2020. Examples of groups to prioritize for screening testing These examples can guide development of local recommendations to prioritize select groups for screening testing, taking into account feasibility and costs. The FDA has collaborated with stakeholders to better understand the public health impact of new SARS-CoV-2 variants and their impact on test performance, has been routinely monitoring publicly available databases, and has coordinated efforts to evaluate the impact of new virus variants on tests that have received Emergency Use Authorization EUA. D Representative projection of RNP hexons assembling into a spherical virus and tetrahedrons into an ellipsoidal virus. Results• Testing employees in a workplace setting• Human-to-human transmission of the virus has been confirmed in all these regions. Infection, Genetics and Evolution: 104812. The results of some of these studies are described below. "Study claiming new coronavirus can be transmitted by people without symptoms was flawed". Potential Impact: Since this test is designed to detect multiple genetic targets, the overall test sensitivity should not be impacted. Although such sites are a common naturally-occurring feature of other viruses, including some members of the genus and other genera of coronaviruses, SARS-Cov-2 is unique among members of its subgenus for such a site. Due to the low SARS-CoV-2 infection rate among the participants, the study was underpowered to detect a prophylactic benefit of hydroxychloroquine. C Ultrastructure of the RNP hexon and tetrahedron assemblies. from the original on 8 March 2020. As of August 24, 2020• Still others, especially those who had severe illness, are left with lingering pulmonary abnormalities. There was no statistical difference between the study arms in the incidence of either PCR-confirmed or symptomatically compatible COVID-19, which was 18. With these coordinated cohorts and datasets, we will better understand the adult and pediatric population burden of PASC, as well as its biological bases and clinical spectrum; identify ways to improve recovery after SARS-CoV-2 infection; and potentially test interventions to prevent long-term disability from SARS-CoV-2 infection. Manufacturer: Mesa Biotech Inc. If local or state clinical laboratories have access to quick turnaround whole genome sequencing services, such as those using the EUA-authorized , these labs should consider further characterizing the specimen with genetic sequencing when this pattern is identified. Viral has also been found in and semen from infected individuals. Study Population• There was no statistical difference in the incidence of confirmed infection between the hydroxychloroquine and control arms 5. Testing students, faculty, and staff in a school or university setting• Surprisingly, even some who were asymptomatic in the acute phase of SARS-CoV-2 infection ended up with symptoms in the post-acute phase. Use a to assist in selecting testing sites. The RaTG13 virus sequence is the closest known sequence to SARS-CoV-2. Local Resolution and Fourier Shell Correlation FSC Curves, Related to Figure 2 A, B Maps of prefusion S in RBD down or one RBD up conformations are colored by their local resolution ranging between 7. Many recoveries from both confirmed and untested infections go unreported, since some countries do not collect this data, but some people have recovered from confirmed infections. , a negative test in persons with symptoms or a positive test in persons without symptoms with a laboratory-based NAAT. People undergoing testing should on• The study was conducted at seven institutions in the United States between March and August 2020. Maurer L, Peris D, Kerl J, Guenther F, Koehler D, Dellweg D. Association of State-Issued Mask Mandates and Allowing On-Premises Restaurant Dining with County-Level COVID-19 Case and Death Growth Rates — United States, March 1-December 31, 2020. 2021;• pdf• The virus subsequently spread to all provinces of China and to more than 150 other countries across the world. Adverse events that are associated with the use of hydroxychloroquine, including gastrointestinal symptoms and rash, occurred in 112 participants: 66 participants 16. from the original on 23 February 2020. Other studies have suggested that the virus may be as well, with potentially being able to transmit the virus. Indirect contact via is another possible cause of infection. 2012156117• 20-1015• from the original on 17 February 2020. These examples are not listed in a priority order. 25 Additional studies of ivermectin for SARS-CoV-2 are ongoing. A second study of 14 hospitals in Vietnam during 2015 found that cloth masks were inferior to surgical masks for protection against clinical upper respiratory illness or laboratory-confirmed viral infection. Considerations When Testing SARS-CoV-2 testing may be incorporated as part of a. Among all domains, the central helical CH region is the best resolved, as evidenced by tubular densities of the alpha-helix bundles. Despite recent advances in the structural elucidation of SARS-CoV-2 proteins, the detailed architecture of the intact virus remains to be unveiled. This is especially important when community risk or transmission levels are substantial or high. In addition, there is potential impact on performance of the test due to a genetic mutation at positions 28877-28878 AG to TC in patient samples. Testing people as a result of contact tracing efforts• Screening testing can improve detection of SARS-CoV-2. Oberholzer M, Febbo P 19 February 2020. Interpretation There was no clinical benefit of administering hydroxychloroquine 600 mg per day for 8 weeks as PrEP to health care workers who were exposed to patients with COVID-19. Practice advisory: vaccinating pregnant and lactating patients against COVID-19. Department of Health and Human Services has race and ethnicity data to health departments, in addition to other data elements, for individuals tested for SARS-CoV-2 or diagnosed with COVID-19. , diagnostic, screening , analytic performance of the test within the context of the level of community transmission, need for rapid results, and other considerations See Table 1. Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers : A Randomized Controlled Trial. Test Name Link to EUA :• Participants were informed of their allocated study arm after being randomized to the intervention or control arm and signing a consent form. Results: SARS-CoV-2 replicates in the upper and lower respiratory tract. Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences, University of Oxford. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Bracis C, Burns E, Moore M, Swan D, Reeves DB, Schiffer JT, Dimitrov D. Mandelbaum RF 19 February 2020. Compared to placebo, hydroxychloroquine was associated with an increased risk of mostly mild adverse events. The COVID-19 Treatment Guidelines Panel the Panel recommends against the use of any drugs for SARS-CoV-2 pre-exposure prophylaxis PrEP , except in a clinical trial AIII. Braun, Elisabeth; Sauter, Daniel 2019. Zhang, Tao; Wu, Qunfu; Zhang, Zhigang April 2020. While larger studies are needed, the researchers note that their findings suggest that the T cell response in convalescent individuals, and most likely in vaccinees, are largely not affected by the mutations found in these three variants, and should offer protection against emerging variants. ACIP considers disease epidemiology, burden of disease, vaccine efficacy and effectiveness, vaccine safety, the quality of the available evidence, and potential vaccination implementation issues. 16 Study Population• The current estimate for the infection's fatality rate is 0. 14 Households were randomized to receive oral hydroxychloroquine 400 mg once daily for 3 days, followed by hydroxychloroquine 200 mg once daily for an additional 11 days, or oral ascorbic acid 500 mg once daily for 3 days, followed by ascorbic acid 250 mg once daily for 11 days. Screening helps to identify unknown cases so that measures can be taken to prevent further transmission. , essential and frontline workers, people living in rural or frontier areas who have experienced a disproportionate burden of COVID-19. Bulletin of the World Health Organization. The investigators noted that the simulations that were used to determine the hydroxychloroquine dose for the study predicted much higher drug concentrations than the observed levels. from the original on 20 January 2020. 18 for the once-weekly hydroxychloroquine arm and 0. D The postfusion S structure fitted with PDB: 6XRA, displaying densities of N1098, N1074, N1158, N1173, and N1194. Bottom right: statistics of the dimension of SARS-CoV-2 viral envelopes. Be aware that molecular tests that use multiple genetic targets to determine a final result are less likely to be impacted by increased prevalence of genetic variants. 2 Health care providers should follow the Centers for Disease Control and Prevention CDC recommendations for infection control and appropriate use of personal protective equipment PPE. When the researchers looked at test results 90 or more days after the initial antibody test when any coronavirus detected by NAAT is likely to reflect a new infection rather than continued virus shedding from the original infection , only about 0. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Face coverings and respiratory tract droplet dispersion. In communities with a higher proportion of racial and ethnic minority populations and by COVID-19, health departments should ensure there is timely and equitable access to and availability of testing with fast result return, especially when the level of community transmission is substantial or high. The study was stopped early. An example of surveillance testing is. Yao X, Ye F, Zhang M, et al. Viral genetic sequence data can provide critical information about whether viruses separated by time and space are likely to be epidemiologically linked. Depending on the time when someone was infected and the timing of the test, the test might not detect antibodies in someone with a current infection. Open Forum Infectious Diseases DOI: 10. PMCID:• Short turnaround time approximately 15 minutes When performed at or near POC, allows for rapid identification of infected people, thus preventing further virus transmission in the community, workplace, etc. Navy Service Members — USS Theodore Roosevelt, April 2020. This information is intended for use by healthcare providers and public health professionals and those organizing and implementing testing in non-health care settings such as schools, workplaces, and congregate housing. Transmission was initially assumed to occur primarily via from coughs and sneezes within a range of about 1. There was an average window of 2 days between the time of the most recent exposure to the index people and the time the study drugs were administered. from the original on 15 May 2020. "Wuhan seafood market may not be source of novel virus spreading globally". Positive and negative predictive values of NAAT and antigen tests vary depending upon the pretest probability. Viral Filtration Efficiency of Fabric Masks Compared with Surgical and N95 Masks. Discussion on testing of vaccinated individuals and interpretation of test results• 351, originally found in the Republic of South Africa; and B. D Compositional analysis of the surface glycans. Leffler 52 169 countries Jan—May County population Mask wearing by tradition, mandate, or recommendation Duration of mask wearing by the public was negatively associated with per-capita mortality from COVID-19. 7,8 Cloth masks not only effectively block most large droplets i. Alsved M, Matamis A, Bohlin R, et al. Its viral evolution is slowed by the capability of its replication machinery. Viral tests can also be used as screening tests to reduce the transmission of SARS-CoV-2 by identifying infected persons who need to from others. [ ] On 11 February 2020, the announced that according to existing rules that compute hierarchical relationships among coronaviruses based on five of nucleic acids, the differences between what was then called 2019-nCoV and the virus from the 2003 SARS outbreak were insufficient to make them separate. "Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins". Its RNA sequence is approximately 30,000 in length, relatively long for a coronavirus. Xpert Xpress SARS-CoV-2, Xpert Xpress SARS-CoV-2 DoD, Xpert Omni SARS-CoV-2• Xiao K, Zhai J, Feng Y, Zhou N, Zhang X, Zou JJ, Li N, Guo Y, Li X, Shen X, Zhang Z, Shu F, Huang W, Li Y, Zhang Z, Chen RA, Wu YJ, Peng SM, Huang M, Xie WJ, Cai QH, Hou FH, Chen W, Xiao L, Shen Y July 2020. Fischer EP, Fischer MC, Grass D, Henrion I, Warren WS, Westman E. Nogrady B 18 November 2020. 8168• Surveillance testing is performed on de-identified specimens, and thus, results are not linked to individual people. Information for the general public on SARS-CoV-2 testing is also.。
Molecular Architecture of the SARS
。
Science Brief: Community Use of Cloth Masks to Control the Spread of SARS
。
Epidemiology of SARS
。
Tackling the Mysteries of the Long
。
Tackling the Mysteries of the Long
。
- 関連記事
2021 www.proinnovate.co.uk


.jpg)